【Hot data】 Resmetirom (MGL-3196) evaluation data available in STAMTM model
As you may already know, the United States Food and Drug Administration (FDA) announced the approval of Rezdiffra (resmetirom), developed by Madrigal Pharmaceuticals, as the first treatment for MASH.
If you are developing a MASH treatment in this situation, you have the possibility to utilize the STAM™-HCC/IO+ model and get the most out of the evaluation for the effectiveness of the compounds.
When considering the launch of a compound, it is extremely important that the results in humans and mice are consistent, from the perspective of reproducing the pathology and mechanism.
We have experience evaluating resmetirom in our STAM™-HCC/IO+ model, and we would like to introduce the results in mice for analysis items that were also evaluated in clinical trials.
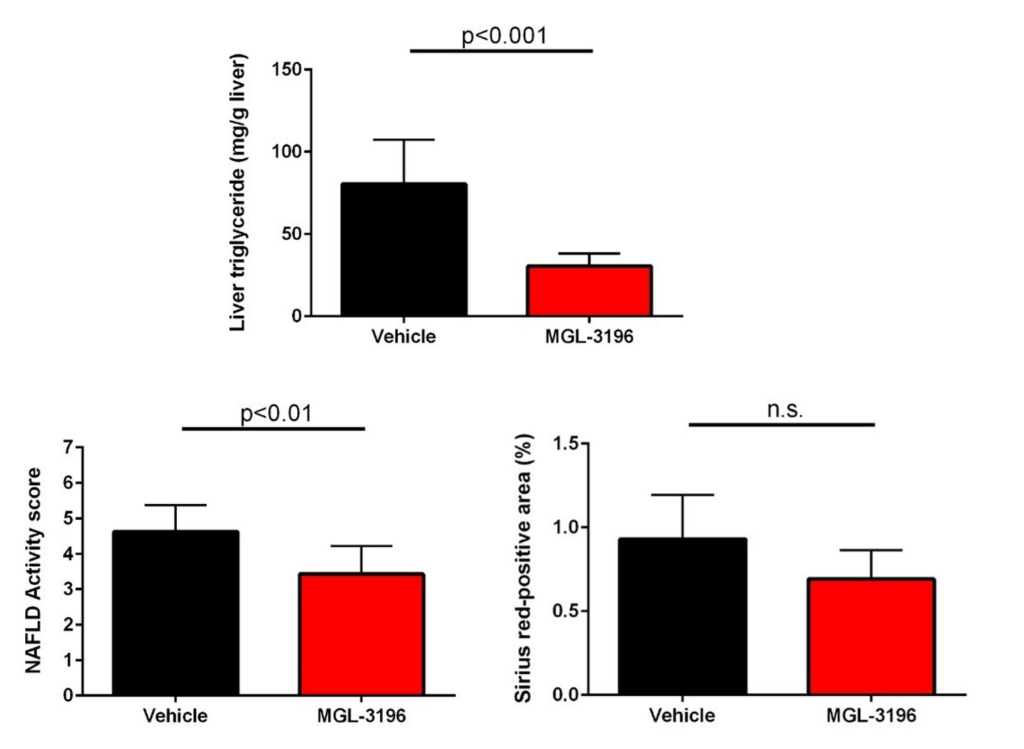
As mentioned above, when considering the mechanism of action of resmetirom, improvements in important lipid parameters, as well as the NAFLD activity score and fibrosis, which was set to be the primary endpoint in clinical trials, have been observed.
Thus, the STAM™-HCC/IO+ model has been shown to show similar improvements in the analysis items evaluated in clinical trials with the approved resmetirom.
More than 15 compounds using the STAM™-HCC/IO+ model have progressed to clinical trials so far, making it a model with extremely high reproducibility of pathological conditions.
In addition, we have conducted over 800 drug efficacy evaluation tests using the STAM™-HCC/IO+ model and gained vast experience, so we can offer you the best proposal.
Please take advantage of this opportunity to contact us.
